Unveiling The Mystery Of Valence Electrons In SO2: A Comprehensive Guide
Valence electrons in SO2 have been a topic of fascination for chemistry enthusiasts, students, and professionals alike. If you're diving into the world of molecular chemistry, understanding sulfur dioxide (SO2) is essential. This compound plays a crucial role in various chemical reactions and atmospheric processes. But what exactly are valence electrons, and why do they matter? Let’s break it down together!
Imagine valence electrons as the "keys" that unlock chemical bonding. These electrons are the outermost electrons in an atom that participate in chemical reactions. When it comes to SO2, the behavior of these electrons determines how sulfur and oxygen atoms interact. Whether you're a student preparing for an exam or a curious mind exploring the intricacies of chemistry, this article will provide you with all the answers you need.
Before we dive deep into the specifics of SO2, let’s establish a foundation. Chemistry can sometimes feel overwhelming, but once you grasp the basics, everything starts to make sense. Valence electrons are the building blocks of molecular chemistry, and understanding them is like learning the alphabet before writing your first sentence. So, buckle up as we unravel the secrets of SO2!
- Exploring The Best Food Court Midway Airport Has To Offer
- What Happened To Andrew Coleman Flipping Out The Untold Story
What Are Valence Electrons?
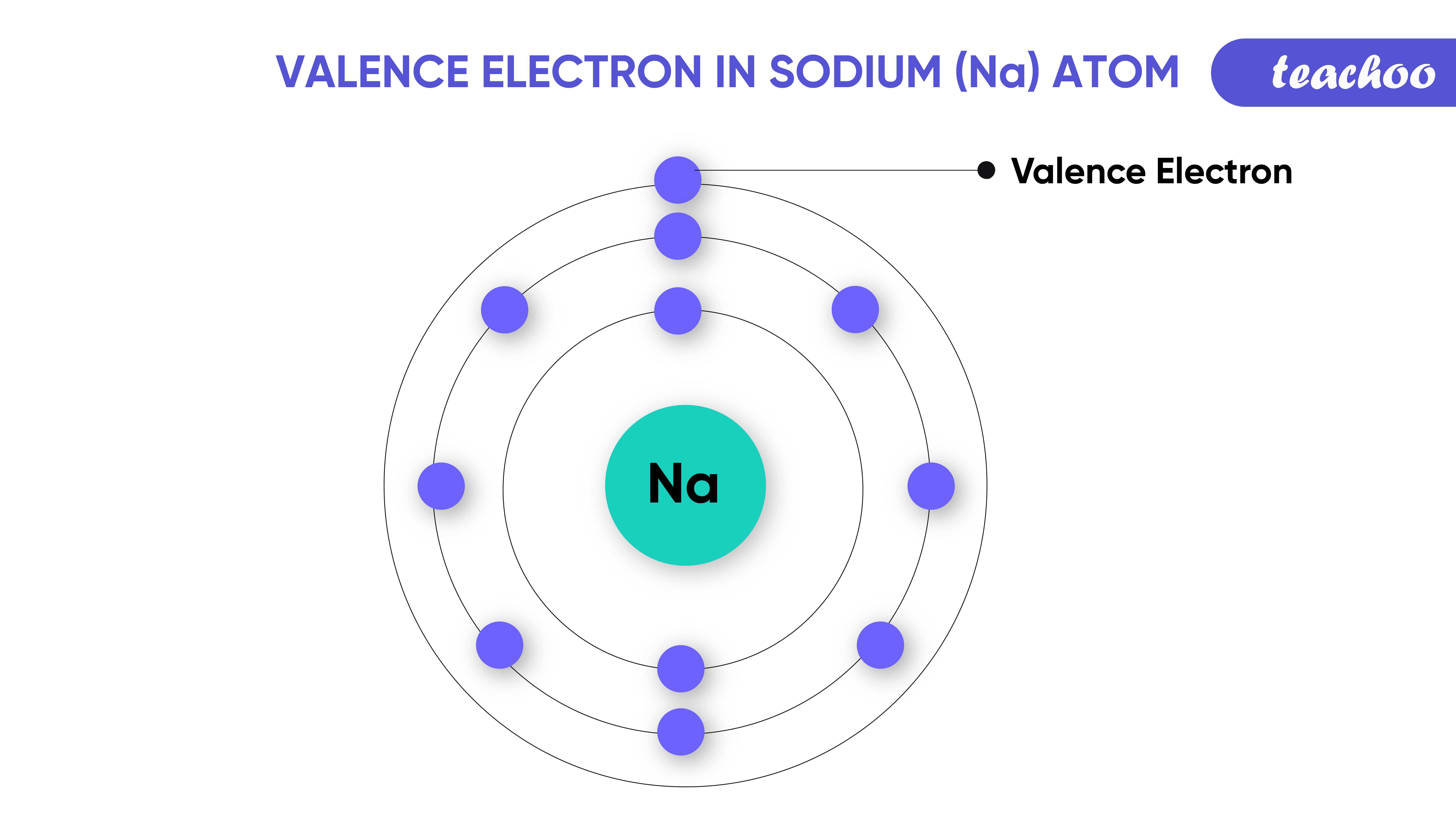
Valence electrons are the electrons located in the outermost shell of an atom. They play a critical role in determining how atoms bond with one another. Think of them as the "social butterflies" of the atomic world—always ready to interact and form connections. Without valence electrons, chemical reactions wouldn't exist, and life as we know it wouldn't function.
In the case of SO2, sulfur and oxygen atoms rely on their valence electrons to create stable bonds. Sulfur, with six valence electrons, and oxygen, also with six, come together in a dance of electron sharing. This sharing results in a molecule that is both fascinating and functional.
Why Do Valence Electrons Matter?
Here’s the deal: valence electrons are the driving force behind chemical reactions. They determine how atoms attract or repel each other, influencing the properties of compounds. For instance, the valence electrons in SO2 contribute to its polarity, reactivity, and overall behavior in different environments.
- Keeganmichael Key Spouse The Untold Story Of Love Laughter And Life
- Chinese In Glastonbury A Cultural Tapestry Unveiled
Let’s break it down further:
- Valence electrons dictate how atoms form covalent, ionic, or metallic bonds.
- They influence the geometry of molecules, such as the bent shape of SO2.
- Understanding valence electrons helps predict the reactivity and stability of compounds.
SO2: The Basics
Sulfur dioxide (SO2) is a compound made up of one sulfur atom and two oxygen atoms. It’s a colorless gas with a distinct, pungent smell. SO2 is not only a key player in industrial processes but also a significant contributor to air pollution. But what makes SO2 unique? Its molecular structure and the role of valence electrons set it apart from other compounds.
In SO2, sulfur acts as the central atom, forming double bonds with each oxygen atom. However, the arrangement of valence electrons creates a bent shape, giving SO2 its characteristic geometry. This shape affects how the molecule interacts with other substances, making it highly reactive.
The Molecular Geometry of SO2
When it comes to SO2, geometry matters. The molecule has a bent shape due to the lone pair of electrons on the sulfur atom. This lone pair repels the bonding pairs of electrons, causing the oxygen atoms to be pushed closer together. The result? A bond angle of approximately 119.5 degrees.
Here’s a quick recap:
- SO2 has a bent shape due to the lone pair on sulfur.
- The bond angle is approximately 119.5 degrees.
- This geometry influences the molecule's polarity and reactivity.
Counting Valence Electrons in SO2
Now, let’s get down to the nitty-gritty. How many valence electrons are in SO2? To figure this out, we need to count the valence electrons for each atom in the molecule.
Sulfur, located in Group 16 of the periodic table, has six valence electrons. Each oxygen atom, also in Group 16, contributes six valence electrons. Add them up, and you get:
Sulfur: 6 valence electrons
Oxygen (2 atoms): 6 x 2 = 12 valence electrons
Total: 6 + 12 = 18 valence electrons
These 18 valence electrons are distributed across the molecule to form stable bonds. But how exactly does this happen? Let’s explore the bonding in SO2.
Valence Electrons and Bonding in SO2
In SO2, sulfur forms double bonds with each oxygen atom. However, the molecule also features a lone pair of electrons on sulfur. This lone pair is crucial because it affects the molecule’s geometry and overall properties.
Here’s how the bonding works:
- Sulfur shares two electrons with each oxygen atom, forming double bonds.
- The remaining electrons form a lone pair on sulfur.
- This arrangement creates a bent shape, making SO2 polar.
Understanding the Lewis Structure of SO2
The Lewis structure of SO2 provides a visual representation of how valence electrons are arranged in the molecule. By drawing the Lewis structure, we can see the bonds and lone pairs clearly.
To create the Lewis structure:
- Place sulfur in the center, surrounded by two oxygen atoms.
- Draw double bonds between sulfur and each oxygen atom.
- Add a lone pair of electrons to sulfur.
This structure reveals the bent shape of SO2 and explains why the molecule is polar. The distribution of valence electrons plays a key role in determining the molecule’s properties.
Resonance Structures of SO2
SO2 also exhibits resonance, meaning it has multiple valid Lewis structures. Resonance occurs when electrons can be distributed in different ways without changing the overall structure of the molecule. In the case of SO2, the double bonds between sulfur and oxygen can shift, creating multiple resonance structures.
Here’s a breakdown of the resonance structures:
- One resonance structure shows a double bond with one oxygen atom and a single bond with the other.
- The second resonance structure swaps the positions of the double and single bonds.
These resonance structures contribute to the stability of SO2 and help explain its reactivity.
The Role of Valence Electrons in SO2’s Reactivity
Valence electrons are the key to understanding why SO2 is so reactive. The lone pair on sulfur makes the molecule polar, which means it can interact with other polar molecules or ions. This reactivity is both a blessing and a curse—SO2 is used in various industrial processes but is also a major contributor to air pollution.
Here’s how valence electrons influence SO2’s reactivity:
- The lone pair on sulfur makes the molecule polar.
- Polarity allows SO2 to dissolve in water, forming sulfuric acid.
- SO2 reacts with other gases, such as oxygen, to form harmful pollutants like sulfur trioxide (SO3).
Environmental Impact of SO2
While SO2 has important industrial applications, its environmental impact cannot be ignored. When released into the atmosphere, SO2 reacts with water vapor to form sulfuric acid, contributing to acid rain. This acid rain can harm ecosystems, damage infrastructure, and pose health risks to humans and animals.
Here’s the bottom line:
- SO2 is a major contributor to air pollution.
- It forms sulfuric acid when combined with water vapor.
- Reducing SO2 emissions is crucial for protecting the environment.
Applications of SO2
Despite its negative environmental impact, SO2 has numerous practical applications. It’s used in the production of sulfuric acid, a vital component in many industrial processes. SO2 is also used as a preservative in the food and wine industries, where it helps prevent oxidation and spoilage.
Here’s a look at some of the key applications:
- Production of sulfuric acid.
- Food and wine preservation.
- Water treatment and disinfection.
Challenges and Solutions
While SO2 is valuable in many industries, its environmental impact poses significant challenges. Reducing SO2 emissions is a top priority for governments and organizations worldwide. Technologies such as scrubbers and catalytic converters are being developed to minimize SO2 release into the atmosphere.
Here’s what’s being done:
- Scrubbers capture SO2 from industrial emissions.
- Catalytic converters reduce SO2 production in vehicles.
- Alternative methods are being explored to replace SO2 in certain applications.
Conclusion: The Importance of Valence Electrons in SO2
In conclusion, valence electrons in SO2 play a critical role in determining the molecule’s structure, reactivity, and applications. From its bent shape to its polarity, the behavior of valence electrons shapes the properties of this fascinating compound. Whether you’re studying chemistry or working in an industry that relies on SO2, understanding its valence electrons is essential.
So, what’s next? If you’ve enjoyed this deep dive into SO2, why not explore other molecules and their valence electrons? Leave a comment below, share this article with your friends, or check out our other chemistry-related content. Together, we can unravel the mysteries of the molecular world—one valence electron at a time!
Table of Contents
- What Are Valence Electrons?
- SO2: The Basics
- Counting Valence Electrons in SO2
- Understanding the Lewis Structure of SO2
- The Role of Valence Electrons in SO2’s Reactivity
- Applications of SO2
- Contact Comed The Ultimate Guide To Understanding Managing And Preventing It
- 24 May Star Sign Discover The Zodiac Sign Traits And Celestial Secrets
How to find Valency? What are valence electrons? Teachoo

How to find Valency? What are valence electrons? Teachoo
how to get the lewis dot structure of SO2 using the formula for (shared